Mechanicalmolecular mechanical QMMM methods. The enzyme is active over a broad pH range 6090.
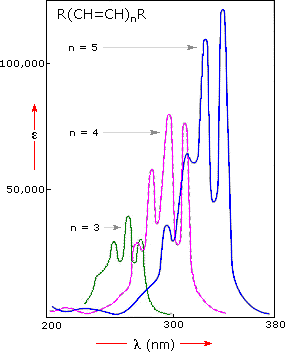
Uv Visible Spectroscopy
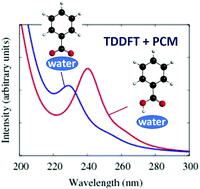
Absorption Spectra Of Benzoic Acid In Water At Different Ph And In The Presence Of Salts Insights From The Integration Of Experimental Data And Theoretical Cluster Models Physical Chemistry Chemical Physics

Q8vmwglub3xrlm
As the dielectric constant of a solution decreases the magnitude of the electrostatic interactions between charged species increases.
Uv absorbance of molecular species and ionic species. DNA hybridization was the first DNA-based technique proposed for the molecular discrimination of Eimeria parasites Shirley 1994b. Agarose is a linear polymer with a molecular weight of about 120000 consisting of alternating D-galactose and 36-anhydro-L-galactopyranose linked by α-13 and β-14 glycosidic bondsThe 36-anhydro-L-galactopyranose is an L-galactose with an anhydro bridge between the 3 and 6 positions although some L-galactose units in the polymer may not contain the bridge. Commonly known as immunogenic cell death ICD.
Molecular mass10 14307 Da amino acid sequence Isoelectric point pI11 1135 Extinction co-efficients. 100 μLwell was added the absorbance was measured at 450 nm using a. The carboxyl end D.
Figure b most likely represents a molecular compound because discrete molecules are present. We would like to show you a description here but the site wont allow us. 213 Figure a most likely represents an ionic compound because there are no discrete molecules only a regular array of two different chemical species ions.
8687 The limitations of SEC have been well documented. Measuring reactive oxygen species with SpectraMax microplate readers. The RNeasy MinElute Cleanup Kit provides high-quality total RNA free from impurities or enzymatic inhibitors with A 260 A 280 ratios of 1921 see figure High-quality RNA.
Ionic skins are of interest for a. Absorption of right circular polarized light vs. E12815 nm12 264 in 01 M potassium chloride EmM 280 nm13 36 Optimal pH.
UVvis absorbance spectra were collected on a Jasco V670 spectrophotometer equipped with a deuterium D2 lamp 190350 nm for use in UV a halogen lamp 3302700 nm for use in UVNIR and an integrating sphere ILN-725 with a working wavelength range of 2202200 nm. The energy required for the transition depends mostly on the extent of conjugation ie. In 1760 Lambert quoted the Bougers discovery in his Photometria which states that the absorbance of a sample is directly proportional to the path length of light.
ICD is characterized by the emission of danger-associated molecular patterns DAMPs that serve to recruit immune cells to the site of the tumor. If the data is collected in a transmission mode the y-axis should be in units of Transmittance T or absorbance abs. The number of consecutive pi bonds.
Species C is unique to the NOHA reaction and has absorbance maxima at 440547 and 580 nm identical to the spectrum of the ferric hemeNO complex of nNOS. Methods using UV-visible spectroscopy. Quantitative analysis method developing for determining an unknown concentration of a species by absorption spectrometry.
Quantitation of protein using UV absorbance is a rapid. 214 a HF is an acid. At the cellular level specific ROS can be individually assessed from tissue culture while at the animal level typically the effects of oxidative stress are measured from blood product eg.
RNA amounts corresponding to less than one cell as little as 1 pg can be concentrated see figure Concentration of RNA A large amount of RNA up to 45 µg can be purified and is suitable for use in. Which can discern the sequential thermal response of different species 44. Molecules are distinguished from ions by their lack of electrical charge.
The color of metal ion solutions is strongly affected by the presence of other species. At pH 62 maximal activity is. For example Shaik et al have discussed the process of CH hydroxy-lation N-oxidation S-oxidation epoxidation of olefins N-dealkylation in various substrates 910.
The extinction coefficient is a physical property of the molecular bonding chemical structure. Most of the organic compounds of biological interest absorb in the UV-visible range of the spectrum. PH ionic strength solvent temperature etc and secondly because it may be important to choose conditions which will not adversely affect the molecular structure of.
In the kinetic theory of gases the term molecule is often used. 100 or 1. In a widely cited example ligand-protected metallic.
Left circular polarized light. A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. To quantitate the nucleic acid concentration dilute the plasmid DNA 1.
In our last post we showed that molecules with C-C pi π bonds absorb light in the UV-visible region which promotes electrons from bonding π orbitals to anti bonding π orbitals. Ideal optical chemosensors should provide selectivity and sensitivity towards a variety of analytes. The measurement of reactive oxygen species is dependent on the analytic target along with the reactive oxygen species in question.
Small molecule-based chromogenic and fluorogenic probes play an indispensable role in many sensing applications. Synthetic accessibility and attractive photophysical properties have made squaraine dyes an enticing platform for the development of chemosensors. Serum or plasma or from urine samples.
If the data is collected using a reflection technique ATR reflection. Total light absorbed by a β-sheet C. A complication to the SEC analysis of a protein drug product can also be the coeleution of excipients such as the non-ionic surfactant polysorbate 80.
In one of its definitions nanochemistry 12 focuses on the intriguing and diverse analogies between molecular-scale and nanosized species. Aiming to study the preferential establishment of ionic interactions tRNA binding was promoted with 10 mM Tris-HCl pH 80. In quantum physics organic chemistry and biochemistry the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
The com-putational studies employ the use of active species Cpd I and utilize theoretical methods in elucidating the mechanism. Lambert did not claim any discovery but he was often credited with it. Agarose gel electrophoresis was used to detect and identify tRNAs species eluted in each chromatographic step while the absorbance of each fraction was measured at 260 nm to infer the recovered RNA levels.
35 Thus the single-turnover experiment reveals that newly formed NO binds to the ferric heme iron before leaving the enzyme. In 1852 August Beer discovered that absorbance is proportional to the sample concentration. Spectrophotometer uses in the Quantitative analysis of Biochemistry practicals.
Total light absorbed by an α-helix vs. A typical protocol consisted of genomic DNA digestion with different restriction enzymes separation through agarose gel electrophoresis blotting and hybridization with DNA probes composed of repetitive regions. D CaO is composed of a metal Ca and nonmetal O and is ionic.
The activity of lysozyme is a function of both pH and ionic strength. To address this issue alternative UV absorbance wavelengths or the intrinsic fluorescence of the protein may be used to advantage. Absorbance by a circular peptide B.
50 depending on the plasmid copy number in TE buffer and measure the absorbance optical density at 260 nm A 260 and 280 nm A 280. To determine the yield DNA concentration should be determined by both UV spectrophotometry at 260 nm and quantitative analysis on an agarose gel. It is clear that one would use absorbance as the scale for any UVVisNIR application that involved quantitation.
The dipole moment of the amino end of an α-helix vs. Absorbance by a linear peptide vs. A Quick Review Of What Weve Learned So Far About UV-Vis.
Ionic liquids were drop cast between two quartz slides.

Uv Vis Spectroscopy An Overview Sciencedirect Topics
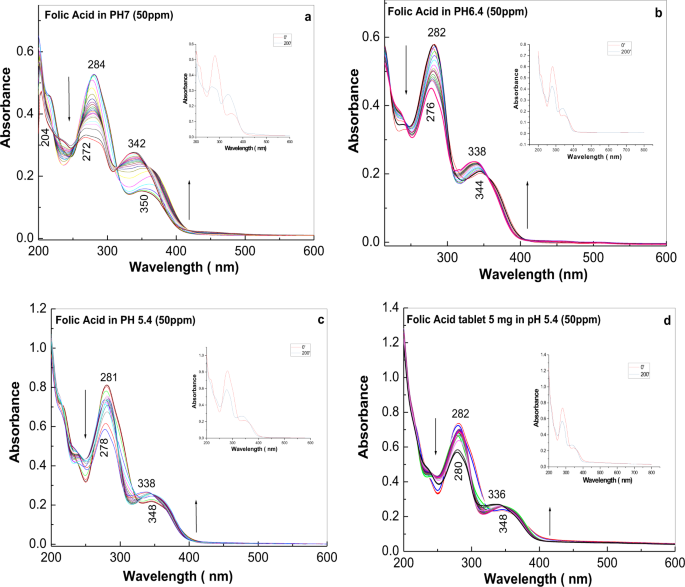
Optical Properties Of Folic Acid In Phosphate Buffer Solutions The Influence Of Ph And Uv Irradiation On The Uv Vis Absorption Spectra And Photoluminescence Scientific Reports

Uv Vis Absorption Spectra A Of 1 1 µm In Ch3cn Solution With Download Scientific Diagram

Ultraviolet Spectrophotometry An Overview Sciencedirect Topics

Diffuse Reflectance Uv Vis Spectrum An Overview Sciencedirect Topics

Uv Visible Absorption Spectrum Of Fenta 0 3 Mm And Chromium Vi Download Scientific Diagram
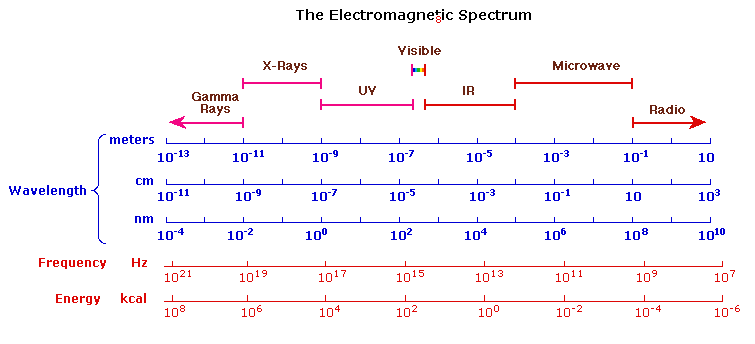
Uv Visible Spectroscopy

The Uv Vis Spectra Of Different Vanadium Standard Solutions With Download Scientific Diagram
